Nobel win for chemistry trio
A university researcher in Australia shares the chemistry Nobel Prize for pioneering new materials that could solve environmental challenges.

University of Melbourne's Richard Robson has been named one of three recipients of the 2025 Nobel Prize in Chemistry.
Professor Robson shares the prize with Susumu Kitagawa of Kyoto University and Omar M Yaghi of the University of California for his work in developing a new class of molecular structures.
Metal-organic frameworks (MOFs) are porous crystalline structures with internal cavities that allow gases and liquids to pass through, offering the capacity to be customised for use in a range of environmental applications.
The Nobel Prize, awarded by the Royal Swedish Academy of Sciences, recognises the trio’s creation of these molecular materials. The SKR11 million (A$1.76 million) prize money will be shared equally between the laureates.
According to the academy, MOFs stand out for their ability to capture carbon dioxide, remove toxic pollutants and harvest water from desert air, among many other applications.
“Metal-organic frameworks have enormous potential, bringing previously unforeseen opportunities for custom-made materials with new functions,” Chair of the Nobel Committee for Chemistry Heiner Linke said.
As a lecturer and researcher at the University of Melbourne since 1966, Professor Robson's early research on molecular construction combined positively charged copper ions with a four-armed molecule that attracted the ions at each end. These produced a well-ordered crystal structure similar to a diamond "filled with innumerable cavities".
"Metal-organic frameworks have enormous potential, bringing previously unforeseen opportunities for custom-made materials with new functions."
Although Robson's metal-organic framework was initially unstable, the Academy said his preliminary work introduced a new way of thinking about molecular construction.
Between 1992 and 2003, Kitagawa and Yaghi separately made a series of discoveries that advanced Robson’s concept, the Academy says.
Kitagawa showed that gases could move in and out of MOFs and predicted that the materials could be flexible. Yaghi later developed a highly stable MOF that could be modified, enabling the design of frameworks with desired properties.
According to the Academy, while numerous MOFs have been developed globally, the materials have only been used on a small scale so far.
However, it says many researchers are now exploring mass production and commercialisation for a range of clean tech applications that include capturing carbon dioxide from industrial sites and removing toxic gases from semiconductor production.
“This is a testament to Professor Robson and others who are inspired and motivated by a deep curiosity about how the world works, and to the institutions that support and enable long-term fundamental research for the benefit of society,” University of Melbourne Vice-Chancellor Professor Emma Johnston said.
Professor Robson described his Nobel win as "a great honour and pleasure”.
He said the concept of molecular architecture had first occurred to him while building physical models for teaching molecular structure. “Some people at the time thought it was a whole load of rubbish. Anyhow, it didn’t turn out that way.”
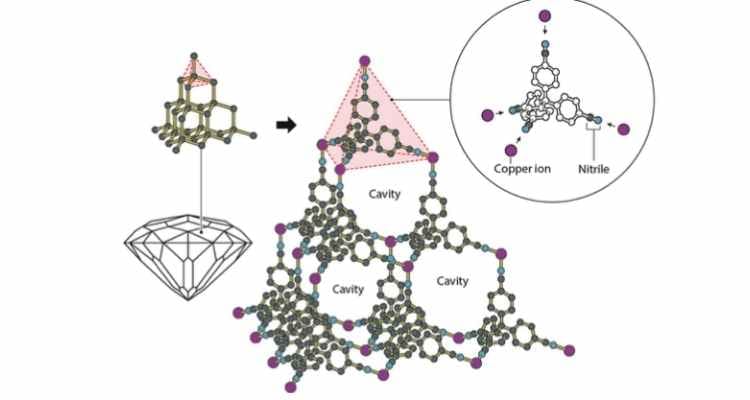
Metal–organic frameworks (MOFs) are described as a new form of molecular architecture. These porous, crystal-like structures are made by linking metal ions with carbon-based molecules. According to researchers, this structure forms internal cavities that can trap, store, or allow the passage of specific chemicals and gases. By varying the building blocks used in the MOFs, chemists can modify the structures to serve many different functions such as removing pollutants, conducting electricity, or enabling chemical reactions, owing to the materials’ ability to selectively target and process specific molecules.





